Products
State of the art: Universal influenza vaccines that target conserved regions of the influenza virus, including the HA stalk domain, the ectodomain of the M2 ion channel, or the internal matrix and nucleoproteins, have achieved solid progress recently. Several promising vaccine candidates are in early clinical trials, with each candidate exploiting conserved regions of the virus. Among those, different strategies directing the antibody response to the conserved HA stalk were evaluated. The most promising approach appears to be sequential vaccination with chimeric HAs (cHAs) with different head domains but the same stalk domain. In a Phase 1 trial, the safety and immunogenicity of the chimeric HA-based approach were demonstrated. Sequential vaccination with cHAs with different head domains but the same stalk domain caused high anti-stalk antibody titres in all experimental groups. Vaccination with cHA vaccines was acceptably safe in adults while providing strong broad-spectrum functional and long-lasting antibodies. Similar results were obtained in a subsequent Phase 1/2 trial. These clinical trials focused on proof of concept and only considered type A/group 1 influenza viruses. However, the researchers are currently developing group 2 and B cHAs with the goal of a trivalent vaccine enabling protection for a significantly wider pool of seasonal influenza viruses.
While the FLUniversal consortium partly builds on these observations, it will go far beyond the current state of the art on different levels. A new strategy to direct the antibodies to the stalk region was developed and combined with a live attenuated approach based on deleting the interferon antagonist NS1. Other objectives of the project are to define new correlates of protection and develop a clinical challenge model that closely mimics natural human infection. Finally, the vaccine candidates will be produced in an innovative production system based on Vero cells. The core of our project is combining two highly innovative concepts:
1. delNS1 live attenuated vaccines:
Deleting the influenza gene called NS1 is the key technical innovation of DeltaFLU. Deletion of the interferon antagonist NS1 (delNS1) results in the unique ability of DeltaFLU to induce interferon (ref 3) rapidly. Upon intranasal administration, DeltaFLU induces interferon, enhancing the generation of broadly cross-neutralising antibodies in the local mucosa and systemic B-and T-cell mediated immunity. Deletion of NS1 also makes DeltaFLU safe because the genetically modified strains are replication-deficient; they do not produce viral progeny and are not shed by vaccine recipients. These properties were demonstrated in preclinical trials and clinical trials involving 245 volunteers (NCT00724997, NCT00724997, NCT01078701, NCT01369862). While in the past, an efficient delNS1 production process was lacking, we recently developed a method that allows to production delNS1 at an optimal dose (8log10).
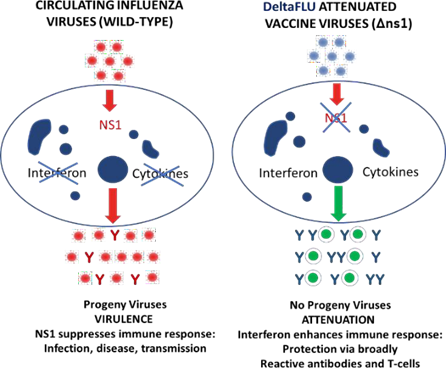
Principle of DeltaFLU vaccine strains. Wild-type influenza virus causes infected cells to synthesise NS1 protein, suppressing the immune response, causing infection, disease and transmission. DeltaFLU viruses lack the NS1 gene (deltaNS1), do not cause the synthesis of NS1, and do not produce progeny; delNS-based vaccines are safely attenuated and highly immunogenic.
2. Sequential HA vaccination:
Recent studies show that antibodies targeting the HA stalk neutralise diverse influenza strains and are broadly protective. In contrast to the head, the HA stalk is highly conserved across influenza strains. While others use HAs that only contain the stalk region or chimeric HAs to direct antibodies to the conserved stalk, we successfully targeted the conserved stalk using wild-type HAs containing similar stalks but different head structures. We achieved it by combining delNS1 vaccine strains with wild-type HAs consisting of different heads and homologous stalks in a prime-boost approach. (Patent application WO2021/127463 Novel prime-boost influenza vaccine). This is an essential improvement since stalk regions by themselves appear to be poorly immunogenic, and chimeric HAs often lack stability and do not grow to sufficient levels to be used in vaccine production. We thus propose to use the delNS1 concept in an innovative immunisation regimen for universal protection from influenza. Ultimately, at the end of the project, we envision FLUniversal providing proof-of-concept data for heterologous protection by a universal influenza vaccine in a new human challenge model for influenza, identifying correlates of protection in clinical and preclinical data, and expanding our molecular understanding of cross-strain protection in preclinical animal models.
The novelty of our solution can be summarised as follows:
At the heart of the innovation is the proprietary DeltaFLU technology. DeltaFLU vaccines are based on genetically modified influenza virus strains in which the NS1 gene has been deleted. NS1 possesses anti-interferon activity. Interferon is a signalling protein produced by the body to activate the immune response. In unmodified (wild-type) influenza viruses, NS1 counteracts the body’s interferon response, allowing the viruses to proliferate and cause disease. The complete and irreversible deletion of NS1 in DeltaFLU vaccine strains achieves two key properties that are highly advantageous:
- Deletion of NS1 makes DeltaFLU vaccines safe – they are unable to replicate and are not shed by the vaccine recipient.
- Deletion of NS1 induces interferon in the nasal passages, achieving a natural “selfadjuvant” effect that induces broadly cross-neutralising antibodies in the nasal passages, creating the first line of defence at the site of viral entry. Interferon activates cytotoxic T cells and antibody-producing B cells for a broadly protective systemic immune response.
The triple-formulated prime-boost immunisation strategy, combined with DeltaFLU technology, will generate a robust immune response of interferon, T cells, and antibodies targeted to HA stalk elements common to all influenza strains. This broad, multifaceted immune response is expected to enable universal protection against all circulating and emerging (drifted and shifted) influenza A and B viruses.
DeltaFLU’s innovative cell-based manufacturing allows a break from the traditional egg-based vaccine production methods, decreases vaccine production time from months to weeks, and reduces waste. The process is economical; the all-in manufacturing cost is projected at ~€3 per immunisation course.
